Overnight Health Care — Presented by Rare Access Action Project — CDC advises Pfizer, Moderna shots over J&J

Welcome to Thursday’s Overnight Health Care, where we’re following the latest moves on policy and news affecting your health. Subscribe here: digital-release.thehill.com/newsletter-signup.
Almost two years into the pandemic, COVID-19 is upending professional sports leagues and teams, leading to a cascade of postponed games and inactive players.
CDC advisers weighed in on the J&J vaccine’s safety issues, and health officials are warning about an omicron wave this winter.
For The Hill, we’re Peter Sullivan (psullivan@digital-release.thehill.com), Nathaniel Weixel (nweixel@digital-release.thehill.com) and Justine Coleman (jcoleman@digital-release.thehill.com). Write to us with tips and feedback, and follow us on Twitter: @PeterSullivan4, @NateWeixel and @JustineColeman8.
Let’s get started.
Health officials recommend mRNA shots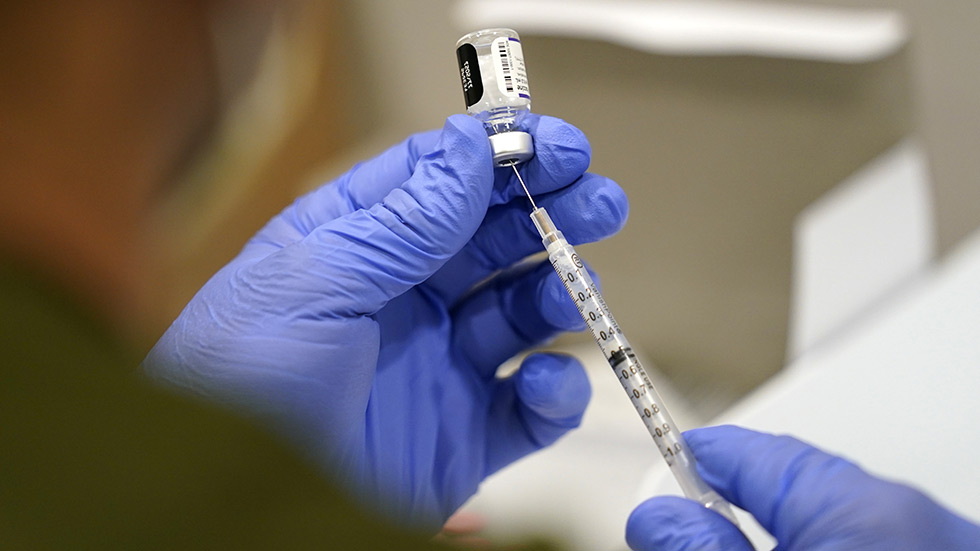
Health providers should offer the Moderna and Pfizer COVID-19 vaccines rather than Johnson & Johnson’s because of an increased risk of blood clots in young and middle-aged women, a panel of advisers to the Centers for Disease Control and Prevention (CDC) said Thursday.
The so-called “preferential” recommendation from the Advisory Committee on Immunization Practices does not necessarily put any limitations or restrictions on the Johnson & Johnson vaccine. Instead, it gives individuals the ability to make a decision for themselves.
The panel voted 15-0 that the mRNA vaccines are preferred to the J&J vaccine, but many members wanted stronger language.
Panelists said the recommendation was not based on the efficacy of the vaccine, but instead on safety. Johnson & Johnson’s shot will still be recommended in cases where people cannot access a different brand or if people want it despite the risk.
Why: The vaccine has been linked to a rare but severe type of blood clot— Thrombosis with Thrombocytopenia Syndrome (TTS)— which led to the product’s pause for 10 days last April. Health officials examined the cases, which were found in just six women out of more than 7 million doses administered.
On Thursday, CDC officials presented new data that showed higher incidences of the clotting disorder. Through Aug. 31, 54 people who received a single dose of the Johnson & Johnson shot were diagnosed with the condition, out of about 14 million shots administered. Overall, the rate of the condition was 3.8 cases per 1 million people; still rare, but more common than previously thought.
Big picture, it wasn’t being used all that much anyway: The panel’s recommendation is another blow to J&J, which has struggled to gain a foothold in the U.S. and is much more popular overseas. Usage dropped considerably after the 10-day pause was lifted, and reports about lower effectiveness also served to tamp down usage.
FDA broadens access to abortion pills

The Food and Drug Administration (FDA) on Thursday expanded access to the abortion pill mifepristone, allowing patients to obtain it by mail instead of requiring in-person visits with specific health care providers.
The FDA said its decision to permanently remove the “in-person dispensing requirement” would “reduce burden on patient access and the health care delivery system.”
Through this action, patients can receive a prescription for mifepristone after a telemedicine appointment and have the pills sent directly to them.
Mifepristone is the first of a two-drug medication used to terminate a pregnancy up to 10 weeks and has been authorized since 2000. Patients previously could obtain the second drug misoprostol, which is supposed to be taken up to 48 hours later, with a standard pharmacy prescription.
Earlier this year, the agency declined to enforce the in-person mandate for the duration of the pandemic after several medical groups warned it put patients at unnecessary greater risk of contracting COVID-19.
It all adds to abortion battles that could play out state by state: The FDA’s move comes as the Supreme Court is considering whether to limit abortion rights in a case involving Mississippi’s 15-week ban that poses a direct challenge to the 1973 precedent of Roe v. Wade.
A MESSAGE FROM RAAP
Tell Congress to protect rare disease patients

Right now, politicians need a win, and rare disease patients are going to lose. Congress is putting huge cost increases on the development of treatments for rare diseases – from 400-800%. Learn more.
Fauci: Omicron likely dominant in US soon

It’s going to be a bleak winter ahead, particularly for the unvaccinated, Anthony Fauci warned on Thursday.
He said the omicron variant will likely be dominant in the U.S. in “a few weeks” and warned of the possibility of hospitals being overwhelmed this winter.
He added, though, that people who are vaccinated, and especially those who have their booster shots, will be “relatively well protected, at least against severe disease,” saying he is most worried about the unvaccinated.
The omicron variant “will assume a dominant role very soon, I would imagine within a period of a few weeks to as we go into January,” Fauci, the government’s top infectious disease expert, said during an event hosted by the U.S. Chamber of Commerce Foundation.
He noted that even before omicron is widespread in the U.S., the delta variant is already causing about 120,000 cases and about 1,000 deaths per day, making added pressure from omicron even worse.
“Besides the toll of suffering and death which will inevitably go up if in fact we have that convergence in the winter months of flu and omicron and delta, we could get our hospital systems overwhelmed,” Fauci said, noting that health workers are already exhausted after almost two years of fighting the pandemic.
The better news: There has been some reassuring news, though, that people who get their booster shots get restored protection against omicron, and that people with two shots still could have protection at least against severe disease.
That means the risk is widely variable depending on someone’s vaccination status.
“With omicron breathing down our back, things could get really bad, particularly for the unvaccinated,” Fauci said. “The vaccinated and those who are boosted I believe will be relatively well protected, at least against severe disease.”
The Biden administration has emphasized that boosters are the best response to the new variant, and has dismissed the idea of further business closures.
BIDEN: WINTER OF ‘SEVERE ILLNESS AND DEATH’ FOR UNVACCINATED
President Biden on Thursday warned of a winter of “severe illness and death” for unvaccinated Americans as coronavirus cases spike across the country.
Biden, in a meeting with medical advisers and Vice President Harris to discuss the pandemic, said the country was in a better position to deal with the omicron variant of COVID-19 because of steps taken to limit travel and increase access to boosters.
“But it’s here now and it’s spreading, and it’s gonna increase,” Biden said of the omicron variant, which experts think is more contagious than previous strains.
“For unvaccinated, we are looking at a winter of severe illness and death… for themselves, their families and the hospitals they’ll soon overwhelm,” Biden continued. “But there’s good news. If you’re vaccinated, and you have your booster shot, you’re protected from severe illness and death, period.”
Biden stressed the effectiveness of booster shots in guarding against severe illness. He noted nearly 57 million Americans have gotten boosted, which experts have said is one way to guard against severe illness regardless of the variants.
Boosters would help keep the economy and schools open, Biden said.
REGENERON SAYS ANTIBODY THERAPY LOSES POTENCY AGAINST OMICRON
Regeneron Pharmaceuticals said on Thursday that an antibody therapy frequently used in unvaccinated COVID-19 patients has “diminished” potency against the omicron variant.
“While Regeneron’s currently authorized REGEN-COV antibodies have diminished potency against Omicron, they are active against Delta, which currently is the most prevalent variant in the U.S.,” Regeneron said in an announcement.
The biotechnology company, however, said that its monoclonal antibodies were still active in patients who had either the omicron or delta variant.
“Regeneron has confirmed that multiple ‘next generation’ monoclonal antibodies from its large collection of fully human monoclonal antibodies targeting SARS-CoV-2 are active against the Omicron (B.1.1.529) and Delta (B.1.617.2) variants, as well as against the other variants of concern,” Regeneron said. “Pending regulatory discussions, we anticipate entering the clinic in the first quarter of 2022.”
A MESSAGE FROM RAAP
Tell Congress to protect rare disease patients

Right now, politicians need a win, and rare disease patients are going to lose. Congress is putting huge cost increases on the development of treatments for rare diseases – from 400-800%. Learn more.
WHAT WE’RE READING
- Understanding omicron’s many mutations (The Washington Post)
- Nurses in crisis over Covid dig in for better work conditions (Kaiser Health News)
- America is not ready for omicron (The Atlantic)
- Soaring infections rattle Europe, fuel dread about holidays (The Associated Press)
STATE BY STATE
- Ohio facing ‘very serious situation’ as COVID cases skyrocket, state’s top doctor says (The Columbus Dispatch)
- New York officials announce some Omicron strategies, including at-home tests by mail. (The New York Times)
- Missouri schools must comply with COVID ruling to win treasurer approval of bond deals (Missouri Independent)
That’s it for today, thanks for reading. Check out The Hill’s health care page for the latest news and coverage. See you tomorrow.
{mosads}
Copyright 2023 Nexstar Media Inc. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed.

